Fine-tuned MOF-74 type variants with open metal sites for high volumetric hydrogen storage at near-ambient temperature
-
Authors :
Dae Won Kim, Minji Jung, Dong Yun Shin, Namju Kim, Jaewoo Park, Jung-Hoon Lee, Hyunchul Oh, and Chang Seop Hong
-
Journal :
Chemical Engineering Journal
-
Vol :
489
-
Page :
151500
-
Year :
2024
Abstract
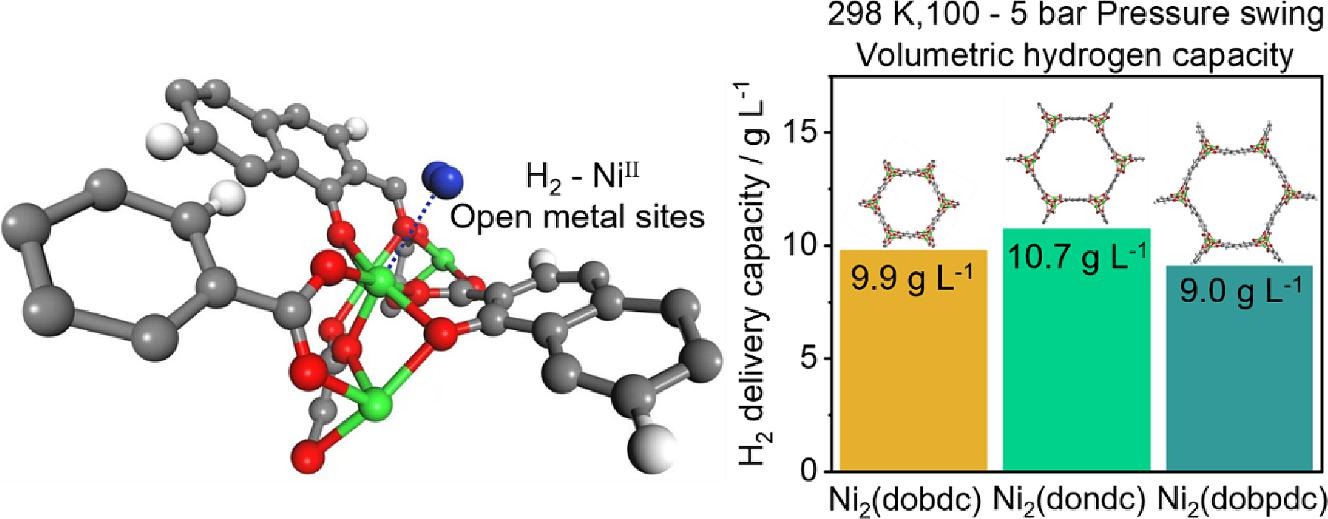
Adsorbent-based hydrogen storage systems offer a potential solution to current challenges in hydrogen storage, particularly those requiring high pressures or cryogenic temperatures. Specifically, the use of metal–organic frameworks (MOFs) featuring open metal sites that strongly adsorb hydrogen represents a promising strategy for near-ambient-temperature hydrogen storage. This study investigates the hydrogen storage properties of M2(dondc) (M = Mg2+, Co2+, and Ni2+), an extended version of MOF-74. Among this series, Ni2(dondc) exhibits the second-highest volumetric hydrogen capacity of 10.74 g L−1 at 298 K under pressure swing adsorption conditions (100 to 5 bar) at ambient temperatures. The superior hydrogen storage performance of Ni2(dondc) is attributed to its highly polarizable Ni open metal sites and a significant heat of adsorption of 12.2 kJ mol−1. These findings are corroborated by temperature-programmed desorption spectroscopy and van der Waals-corrected density functional theory calculations. In addition to its exceptional hydrogen capacity, Ni2(dondc) exhibits robust structural stability and long-term durability, positioning it as a promising candidate for near-ambient-temperature hydrogen storage applications.
















