Atomistic origin of mechanochemical NH3 synthesis on Fe catalysts
-
Authors :
Hong Woo Lee, Ga-Un Jeong, Min-Cheol Kim, Donghun Kim, Sooyeon Kim, Sang Soo Han
-
Journal :
International Journal of Hydrogen Energy
-
Vol :
48
-
Page :
3931-3941
-
Year :
2023
Abstract
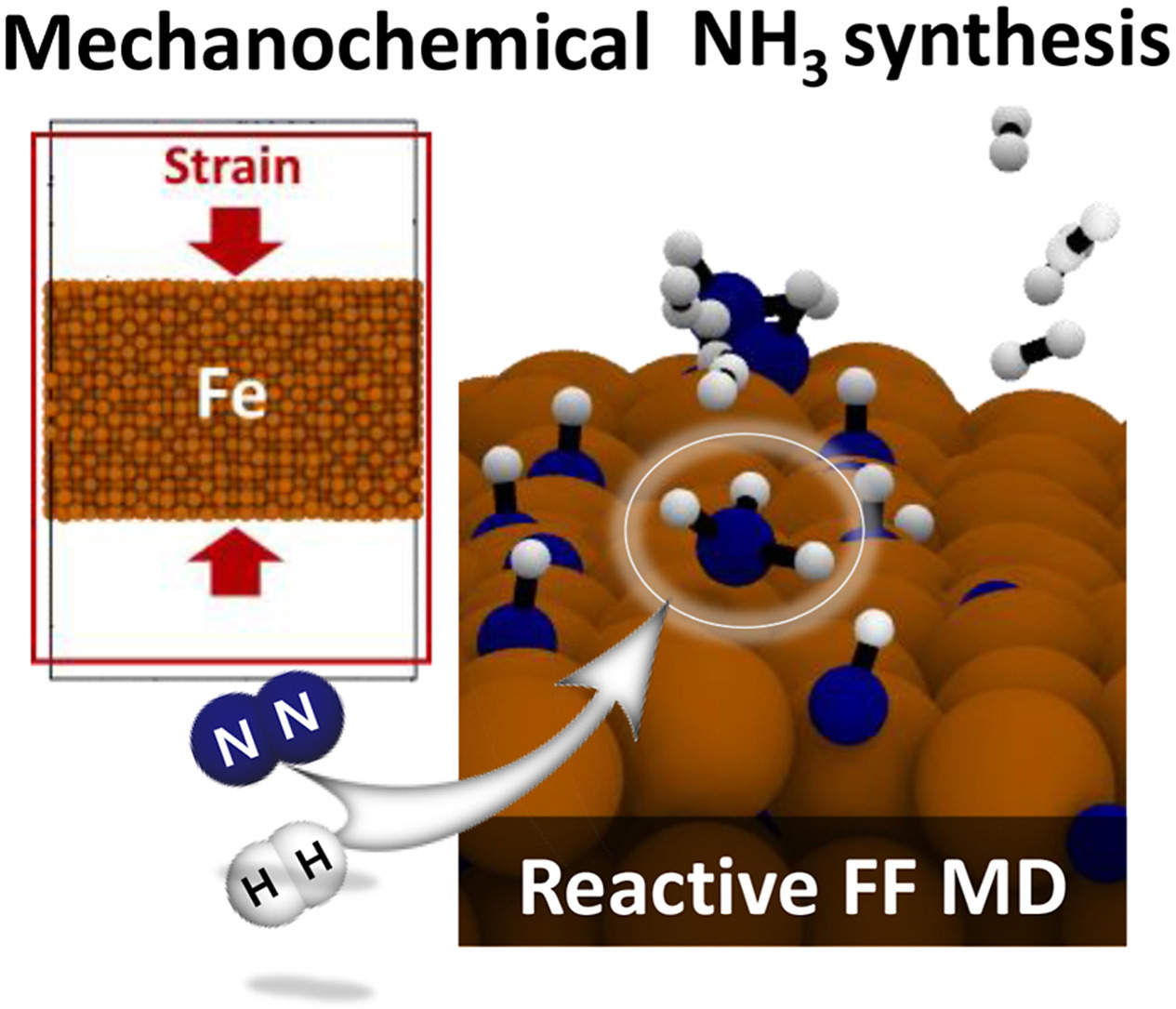
A tremendous amount of ammonia (NH3) has been produced by the Haber-Bosch method during the last 100 years. However, it is imperative to develop a new alternative to produce NH3 in a more environmentally friendly way due to the high energy cost and the large amount of greenhouse gas emissions in the Haber-Bosch method. Although the mechanochemical process has been regarded as an emerging technique, the understanding at the atomic level is limited. Here, we computationally model the mechanochemical ball-milling method by molecular dynamics simulations with the reactive force field (ReaxFF) developed in this work. We find that strain applied to Fe surfaces significantly enhances the N2 dissociation as well as the NHx (x = 1–3) formation on Fe(110), whereas the strain effect is negligible on Fe(111). From the von Mises stress analyses, the applied strain (mechanical) energies transfer the Fe catalysts and N atoms adsorbed on Fe surfaces and then activate NHx formation. Moreover, mechanical strain boosts a new mechanism for NH formation, the rate-determining step, via the direct interaction between the dissociated N atom on the Fe surface and the H2 molecule, which is supported by density functional theory calculations. This study shows that the mechanochemical process is readily operated on Fe catalysts and promising for NH3 synthesis.
















