Theoretical Study of Oxygen Reduction Reaction Mechanism in Metal-Free Carbon Materials: Defects, Structural Flexibility, and Chemical Reaction
-
Authors :
Keunsu Choi, Seungchul Kim
-
Journal :
ACS Nano
-
Vol :
16
-
Page :
16394–16401
-
Year :
2022
Abstract
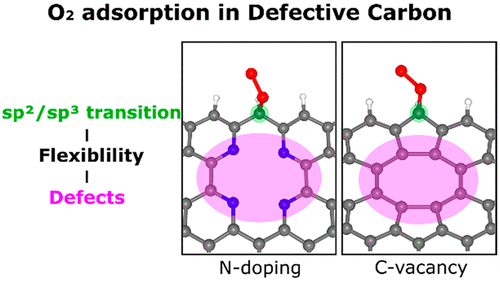
Metal-free carbon materials are attractive Pt-based catalyst alternatives. However, despite efforts, the reaction mechanism remains elusive. Thus, we investigated the role of defects (dopant nitrogen and carbon vacancy) on the catalytic oxygen reduction reaction in a metal-free carbon material focusing on the effect of structural flexibility. Crucially, defects lower the energy barrier for the sp2/sp3 transition of the carbon-centered O2-adsorption sites by releasing structural strain during the reaction. In particular, low-coordinated pyridinic-N displaces from the carbon plane to release the strain, whereas weak C–C bonds around the carbon vacancy change the bond lengths to release the strain. Defects indirectly promote the adsorption of oxygen by enhancing structural flexibility. Thus, the nonlocal structural environment is as critical as the direct interaction between adsorption sites and adsorbate in the chemical reaction. Molecular dynamics simulations reveal that pyridinic-N doping is a facile route to introduce stable catalytic active sites. Overall, our results provide a deeper understanding of chemical processes on defective carbon materials.
















