QM/MM based 3D QSAR models for potent B-Raf inhibitors
슈퍼관리자
2021-05-21
QM/MM based 3D QSAR models for potent B-Raf inhibitors
-
Authors :
Jae Yoon Chung, Hwan Won Chung, Seung Joo Cho, Jung-Mi Hah and Art E. Cho
-
Journal :
J Comput Aided Mol Des
-
Vol :
24
-
Page :
385–397
-
Year :
2010
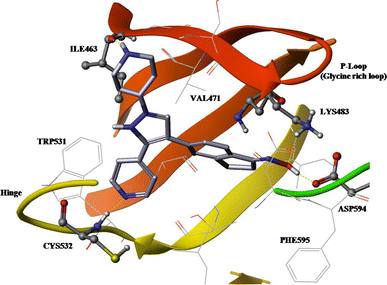
Abstract
Three dimensional (3D) quantitative structure-activity relationship studies of 37 B-Raf inhibitors, pyrazole-based derivatives, were performed. Based on the co-crystallized compound (PDB ID: 3D4Q), several alignment methods were utilized to derive reliable comparative molecular field analysis (CoMFA) and comparative molecular similarity indices analysis (CoMSIA) models. Receptor-guided alignment with quantum mechanics/molecular mechanics (QM/MM) minimization led to the best CoMFA model (q 2 = 0.624, r 2 = 0.959). With the same alignment, a statistically reliable CoMSIA model with steric, H-bond acceptor, and hydrophobic fields was also derived (q 2 = 0.590, r 2 = 0.922). Both models were validated with an external test set, which gave satisfactory predictive r 2 values of 0.926 and 0.878, respectively. Contour maps from CoMFA and CoMSIA models revealed important structural features responsible for increasing biological activity within the active site and explained the correlation between biological activity and receptor-ligand interactions. New fragments were identified as building blocks which can replace R1-3 groups through combinatorial screening methods. By combining these fragments a compound with a high bioactivity level prediction was found. These results can offer useful information for the design of new B-Raf inhibitors.















