Adsorption, Dissociation, and Diffusion of N2 on and in BCC Fe: First-principles Calculations
슈퍼관리자
2021-05-21
Adsorption, Dissociation, and Diffusion of N2 on and in BCC Fe: First-principles Calculations
-
Authors :
S. C. Yeo, S. S. Han, and H. M. Lee
-
Journal :
Physical Chemistry and Chemical Physics
-
Vol :
15
-
Page :
5186-5192
-
Year :
2013
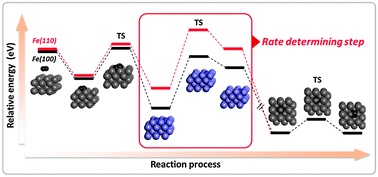
Abstract
We report first-principles calculations of adsorption, dissociation, penetration, and diffusion for the complete nitridation mechanism of nitrogen molecules on a pure Fe surface (bcc, ferrite phase). The mechanism of the definite reaction path was calculated by dividing the process into four steps. We investigated various reaction paths for each step including the energy barrier based on the climb image nudged elastic band (CI-NEB) method, and the complete reaction pathway was computed as the minimum energy path (MEP). The adsorption characteristics of nitrogen (N) and molecular nitrogen (N2) indicate that nitrogen atoms and molecules are energetically favorable at the hollow sites on pure Fe(100) and (110). The dissociation of the nitrogen molecule (N2) was theoretically supported by electronic structure calculations. The penetration of nitrogen from the surface to the sub-surface has a large energy barrier compared with the other steps. The activation energy calculated for nitrogen diffusion in pure bcc Fe was in good agreement with the experimental results. Finally, we confirmed the rate-determining step for the full nitridation reaction pathway. This study provides fundamental insight into the nitridation mechanism for nitrogen molecules in pure bcc Fe.















