Overcoming Metastable CO2 Adsorption in a Bulky Diamine-Appended Metal–Organic Framework
-
Authors :
Bhavish Dinakar, Alexander C. Forse, Henry Z. H. Jiang, Ziting Zhu, Jung-Hoon Lee, Eugene J. Kim, Surya T. Parker, Connor J. Pollak, Rebecca L. Siegelman, Phillip J. Milner, Jeffrey A. Reimer, and Jeffrey R. Long
-
Journal :
Journal of the American Chemical Society
-
Vol :
143
-
Page :
15258-15270
-
Year :
2021
Abstract
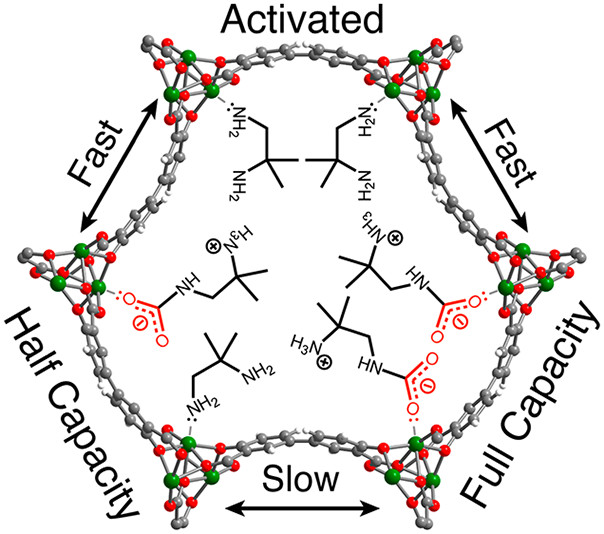
Carbon capture at fossil fuel-fired power plants is a critical strategy to mitigate anthropogenic contributions to global warming, but widespread deployment of this technology is hindered by a lack of energy-efficient materials that can be optimized for CO2 capture from a specific flue gas. As a result of their tunable, step-shaped CO2 adsorption profiles, diamine-functionalized metal–organic frameworks (MOFs) of the form diamine–Mg2(dobpdc) (dobpdc4– = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) are among the most promising materials for carbon capture applications. Here, we present a detailed investigation of dmen–Mg2(dobpdc) (dmen = 1,2-diamino-2-methylpropane), one of only two MOFs with an adsorption step near the optimal pressure for CO2 capture from coal flue gas. While prior characterization suggested that this material only adsorbs CO2 to half capacity (0.5 CO2 per diamine) at 1 bar, we show that the half-capacity state is actually a metastable intermediate. Under appropriate conditions, the MOF adsorbs CO2 to full capacity, but conversion from the half-capacity structure happens on a very slow time scale, rendering it inaccessible in traditional adsorption measurements. Data from solid-state magic angle spinning nuclear magnetic resonance spectroscopy, coupled with van der Waals-corrected density functional theory, indicate that ammonium carbamate chains formed at half capacity and full capacity adopt opposing configurations, and the need to convert between these states likely dictates the sluggish post-half-capacity uptake. By use of the more symmetric parent framework Mg2(pc-dobpdc) (pc-dobpdc4– = 3,3′-dioxidobiphenyl-4,4′-dicarboxylate), the metastable trap can be avoided and the full CO2 capacity of dmen–Mg2(pc-dobpdc) accessed under conditions relevant for carbon capture from coal-fired power plants.
















