Enabling Reversible Redox Reactions in Electrochemical Cells using Protected LiAl Intermetallic as Lithium Metal Anodes
슈퍼관리자
2021-05-21
Enabling Reversible Redox Reactions in Electrochemical Cells using Protected LiAl Intermetallic as Lithium Metal Anodes
-
Authors :
Mun Sek Kim, Deepika, Seung Hun Lee, Min-Seop Kim, Ji-Hyun Ryu, Kwang-Ryeol Lee, Lynden A. Archer, Won Il Cho
-
Journal :
Science Adv.
-
Vol :
5
-
Page :
2375-2548
-
Year :
2019
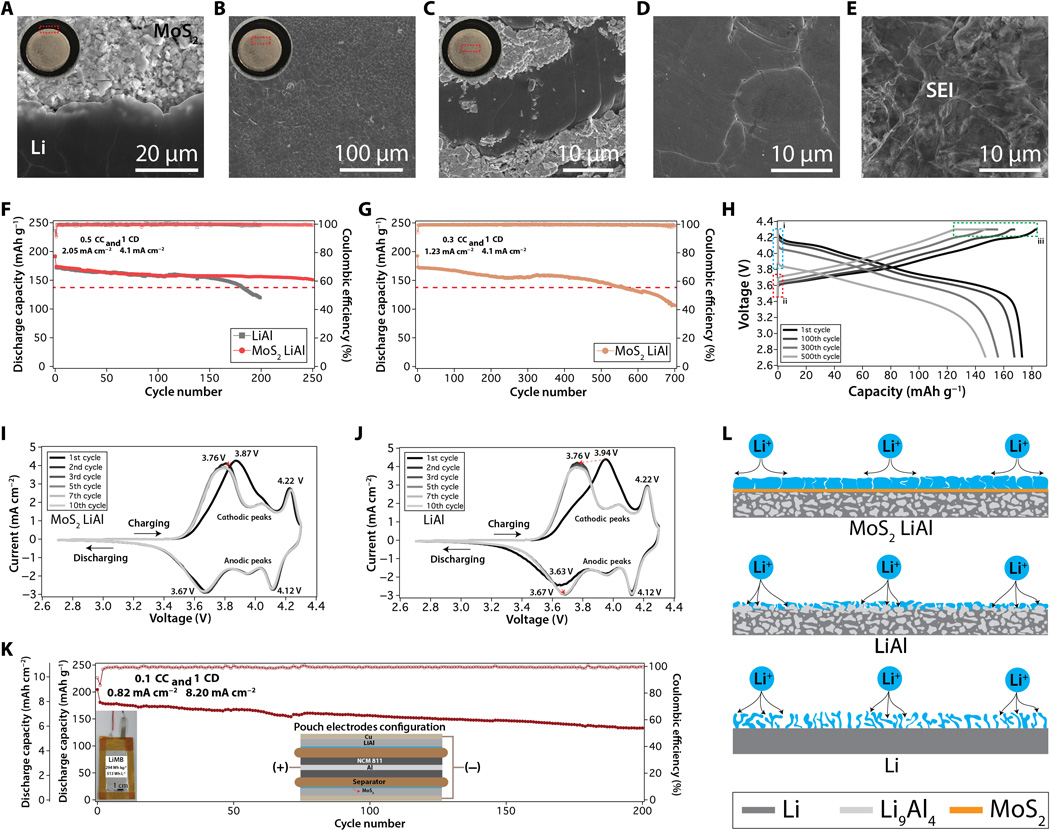
Abstract
Rechargeable electrochemical cells with metallic anodes are of increasing scientific and technological interest. The complex composition, poorly defined morphology, heterogeneous chemistry, and unpredictable mechanics of interphases formed spontaneously on the anodes are often examined but rarely controlled. Here, we couple computational studies with experimental analysis of well-defined LiAl electrodes in realistic electrochemical environments to design anodes and interphases of known composition. We compare phase behavior, Li binding energies, and activation energy barriers for adatom transport and study their effects on the electrochemical reversibility of battery cells. As an illustration of potential practical benefits of our findings, we create cells in which LiAl anodes protected by Langmuir-Blodgett MoS2 interphases are paired with 4.1 mAh cm−2 LiNi0.8Co0.1Mn0.1O2 cathodes. These studies reveal that small- and larger-format (196 mAh, 294 Wh kg−1, and 513 Wh liter−1) cells based on protected LiAl anodes exhibit high reversibility and support stable Li migration during recharge of the cells.