Enhancement of CO2 Binding and Mechanical Properties Upon Diamine Functionalization of M2(dobpdc) Metal-Organic Frameworks
슈퍼관리자
2021-05-21
Enhancement of CO2 Binding and Mechanical Properties Upon Diamine Functionalization of M2(dobpdc) Metal-Organic Frameworks
-
Authors :
Jung-Hoon Lee, Rebecca L. Siegelman, Lorenzo Maserati, Tonatiuh Rangel, Brett A. Helms, Jeffrey R. Long, and Jeffrey B. Neaton
-
Journal :
Chem. Sci.
-
Vol :
9
-
Page :
5197
-
Year :
2018
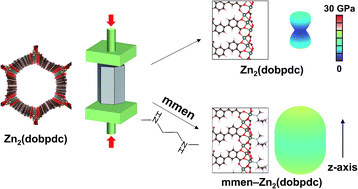
Abstract
The family of diamine-appended metal–organic frameworks exemplified by compounds of the type mmen–M2(dobpdc) (mmen = N,N′-dimethylethylenediamine; M = Mg, Mn, Fe, Co, Zn; dobpdc4− = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) are adsorbents with significant potential for carbon capture, due to their high working capacities and strong selectivity for CO2 that stem from a cooperative adsorption mechanism. Herein, we use first-principles density functional theory (DFT) calculations to quantitatively investigate the role of mmen ligands in dictating the framework properties. Our van der Waals-corrected DFT calculations indicate that electrostatic interactions between ammonium carbamate units significantly enhance the CO2 binding strength relative to the unfunctionalized frameworks. Additionally, our computed energetics show that mmen–M2(dobpdc) materials can selectively adsorb CO2 under humid conditions, in agreement with experimental observations. The calculations further predict an increase of 112% and 124% in the orientationally-averaged Young's modulus E and shear modulus G, respectively, for mmen–Zn2(dobpdc) compared to Zn2(dobpdc), revealing a dramatic enhancement of mechanical properties associated with diamine functionalization. Taken together, our calculations demonstrate how functionalization with mmen ligands can enhance framework gas adsorption and mechanical properties.















