Elucidating CO2 Chemisorption Mechanisms in Diamine-Appended Metal–Organic Frameworks
슈퍼관리자
2021-05-21
Elucidating CO2 Chemisorption Mechanisms in Diamine-Appended Metal–Organic Frameworks
-
Authors :
Alexander C. Forse, Phillip J. Milner, Jung-Hoon Lee, Halle N. Redfearn, Julia Oktawiec, Rebecca L. Siegelman, Jeffrey D. Martell, Bhavish Dinakar, Leo B. Porter-Zasada, Miguel I. Gonzalez, Jeffrey B. Neaton, Jeffrey R. Long, and Jeffrey A. Reimer
-
Journal :
J. Am. Chem. Soc.
-
Vol :
140
-
Page :
18016
-
Year :
2018
Abstract
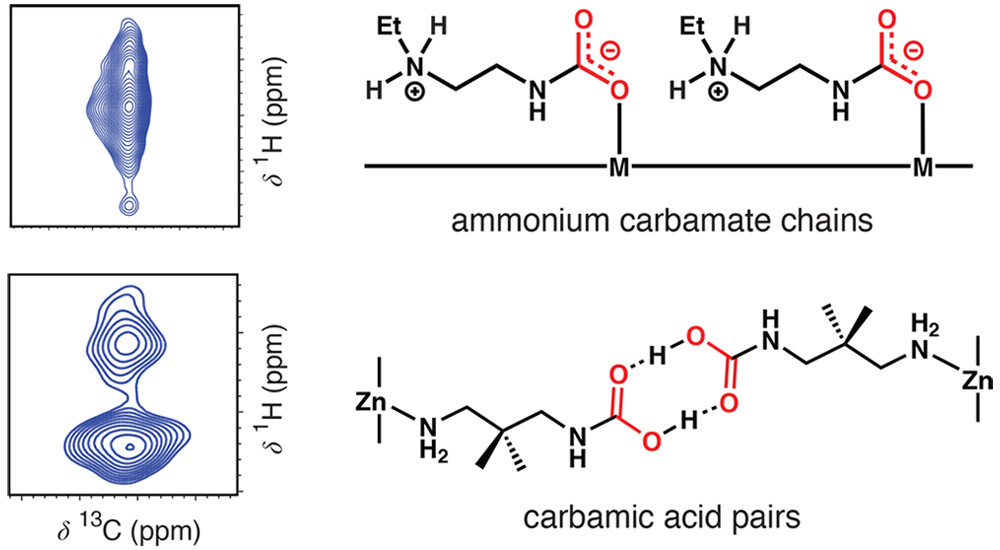
The widespread deployment of carbon capture and sequestration as a climate change mitigation strategy could be facilitated by the development of more energy-efficient adsorbents. Diamine-appended metal–organic frameworks of the type diamine–M2(dobpdc) (M = Mg, Mn, Fe, Co, Ni, Zn; dobpdc4– = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) have shown promise for carbon-capture applications, although questions remain regarding the molecular mechanisms of CO2 uptake in these materials. Here we leverage the crystallinity and tunability of this class of frameworks to perform a comprehensive study of CO2 chemisorption. Using multinuclear nuclear magnetic resonance (NMR) spectroscopy experiments and van-der-Waals-corrected density functional theory (DFT) calculations for 13 diamine–M2(dobpdc) variants, we demonstrate that the canonical CO2 chemisorption products, ammonium carbamate chains and carbamic acid pairs, can be readily distinguished and that ammonium carbamate chain formation dominates for diamine–Mg2(dobpdc) materials. In addition, we elucidate a new chemisorption mechanism in the material dmpn–Mg2(dobpdc) (dmpn = 2,2-dimethyl-1,3-diaminopropane), which involves the formation of a 1:1 mixture of ammonium carbamate and carbamic acid and accounts for the unusual adsorption properties of this material. Finally, we show that the presence of water plays an important role in directing the mechanisms for CO2 uptake in diamine–M2(dobpdc) materials. Overall, our combined NMR and DFT approach enables a thorough depiction and understanding of CO2 adsorption within diamine–M2(dobpdc) compounds, which may aid similar studies in other amine-functionalized adsorbents in the future.















