Understanding Correlation Between CO2 Insertion Mechanism and Chain Length of Diamine in Metal-Organic Framework Adsorbents
-
Authors :
Susan E. Ju, Jong Hyeak Choe, Minjung Kang, Dong Won Kang, Hyojin Kim, Jung-Hoon Lee, Chang Seop Hong
-
Journal :
ChemSusChem
-
Vol :
14
-
Page :
2426-2433
-
Year :
2021
Abstract
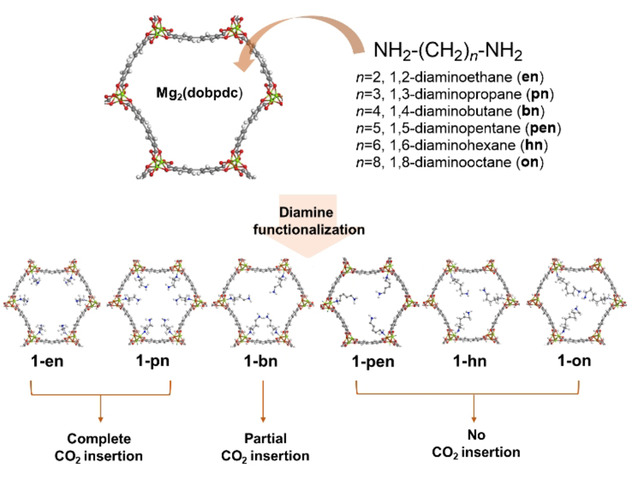
Although CO2 insertion is a predominant phenomenon in diamine-functionalized Mg2(dobpdc) (dobpdc4−=4,4-dioxidobiphenyl-3,3′-dicarboxylate) adsorbents, a high-performance metal-organic framework for capturing CO2, the fundamental function of the diamine carbon chain length in the mechanism remains unclear. Here, Mg2(dobpdc) systems with open metal sites grafted by primary diamines NH2−(CH2)n−NH2 were developed, with en (n=2), pn (n=3), bn (n=4), pen (n=5), hn (n=6), and on (n=8). Based on CO2 adsorption and IR results, CO2 insertion is involved in frameworks with n=2 and 3 but not in systems with n≥5. According to NMR data, bn-appended Mg2(dobpdc) exhibited three different chemical environments of carbamate units, attributed to different relative conformations of carbon chains upon CO2 insertion, as validated by first-principles density functional theory (DFT) calculations. For 1-hn and 1-on, DFT calculations indicated that diamine inter-coordinated open metal sites in adjacent chains bridged by carboxylates and phenoxides of dobpdc4−. Computed CO2 binding enthalpies for CO2 insertion (−27.8 kJ mol−1 for 1-hn and −20.2 kJ mol−1 for 1-on) were comparable to those for CO2 physisorption (−19.3 kJ mol−1 for 1-hn and −20.8 kJ mol−1 for 1-on). This suggests that CO2 insertion is likely to compete with CO2 physisorption on diamines of the framework when n≥5.