Water Enables Efficient CO2 Capture from Natural Gas Flue Emissions in an Oxidation-Resistant Diamine-Appended Metal–Organic Framework
슈퍼관리자
2021-05-21
Water Enables Efficient CO2 Capture from Natural Gas Flue Emissions in an Oxidation-Resistant Diamine-Appended Metal–Organic Framework
-
Authors :
Rebecca L Siegelman, Phillip J Milner, Alexander C Forse, Jung-Hoon Lee, Kristen A Colwell, Jeffrey B Neaton, Jeffrey A Reimer, Simon C Weston, Jeffrey R Long
-
Journal :
J. Am. Chem. Soc.
-
Vol :
141
-
Page :
13171
-
Year :
2019
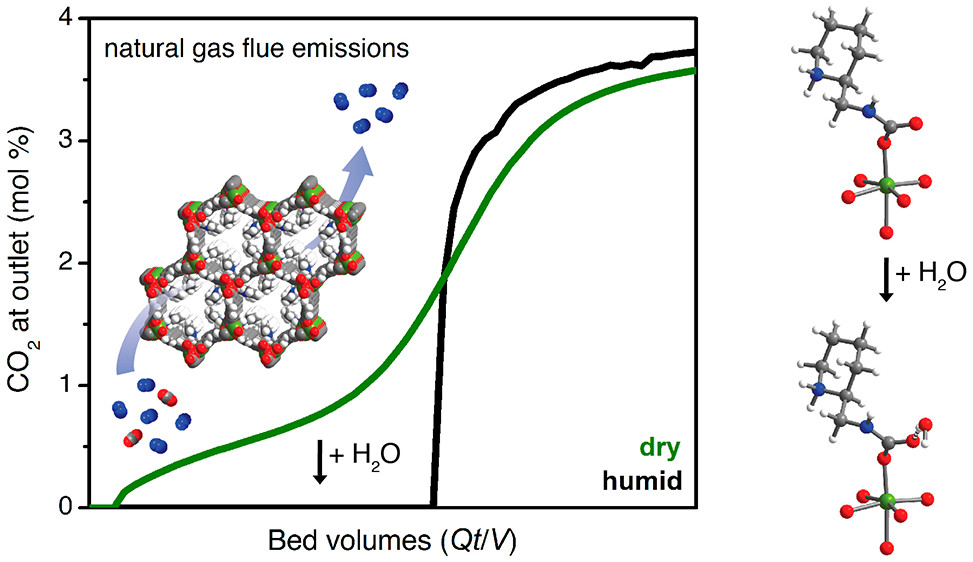
Abstract
Supported by increasingly available reserves, natural gas is achieving greater adoption as a cleaner-burning alternative to coal in the power sector. As a result, carbon capture and sequestration from natural gas-fired power plants is an attractive strategy to mitigate global anthropogenic CO2 emissions. However, the separation of CO2 from other components in the flue streams of gas-fired power plants is particularly challenging due to the low CO2 partial pressure (∼40 mbar), which necessitates that candidate separation materials bind CO2 strongly at low partial pressures (≤4 mbar) to capture ≥90% of the emitted CO2. High partial pressures of O2 (120 mbar) and water (80 mbar) in these flue streams have also presented significant barriers to the deployment of new technologies for CO2 capture from gas-fired power plants. Here, we demonstrate that functionalization of the metal–organic framework Mg2(dobpdc) (dobpdc4– = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) with the cyclic diamine 2-(aminomethyl)piperidine (2-ampd) produces an adsorbent that is capable of ≥90% CO2 capture from a humid natural gas flue emission stream, as confirmed by breakthrough measurements. This material captures CO2 by a cooperative mechanism that enables access to a large CO2 cycling capacity with a small temperature swing (2.4 mmol CO2/g with ΔT = 100 °C). Significantly, multicomponent adsorption experiments, infrared spectroscopy, magic angle spinning solid-state NMR spectroscopy, and van der Waals-corrected density functional theory studies suggest that water enhances CO2 capture in 2-ampd–Mg2(dobpdc) through hydrogen-bonding interactions with the carbamate groups of the ammonium carbamate chains formed upon CO2 adsorption, thereby increasing the thermodynamic driving force for CO2 binding. In light of the exceptional thermal and oxidative stability of 2-ampd–Mg2(dobpdc), its high CO2 adsorption capacity, and its high CO2 capture rate from a simulated natural gas flue emission stream, this material is one of the most promising adsorbents to date for this important separation.















