Mechanistic Investigation of the Catalytic Decomposition of Ammonia (NH3) on an Fe(100) Surface: A DFT Study
슈퍼관리자
2021-05-21
Mechanistic Investigation of the Catalytic Decomposition of Ammonia (NH3) on an Fe(100) Surface: A DFT Study
-
Authors :
S. C. Yeo, S. S. Han, and H. M. Lee
-
Journal :
Journal of Physical Chemistry C
-
Vol :
118
-
Page :
5309-5316
-
Year :
2014
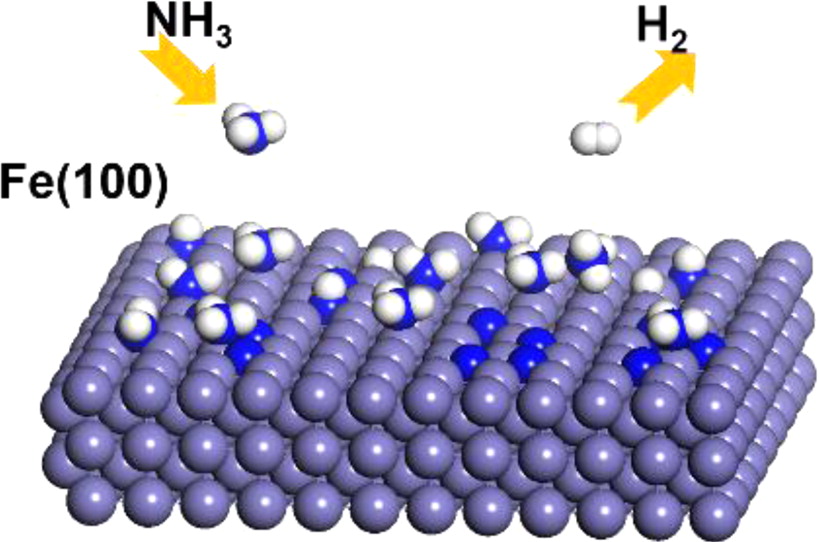
Abstract
Catalytic decomposition of ammonia (NH3) is a promising chemical reaction in energy and environmental applications. Density functional theory (DFT) calculations were performed to clarify the detailed catalytic mechanism of NH3 decomposition on an Fe(100) surface. Specifically, the elementary steps of the mechanism were calculated for the general dehydrogenation pathway of NH3. The adsorption of two types of ammonia dimers (2NH3), locally adsorbed NH3 and hydrogen-bonded NH3, were then compared, revealing that locally adsorbed NH3 is more stable than hydrogen-bonded NH3. By contrast, the dehydrogenation of dimeric NH3 results in a high energy barrier. Moreover, the catalytic characteristics of NH3 decomposition on a nitrogen (N)-covered Fe surface must be considered because the recombination of nitrogen (N2) and desorption have an extremely high energy barrier. Our results indicate that the catalytic characteristics of the NH3 decomposition reaction are altered by N coverage of the Fe surface. This study primarily focused on energetic and electronic analysis. Finally, we conclude that Fe is an alternative catalyst for the decomposition of NH3 in COx-free hydrogen production.















