Theoretical Prediction of the Complexation Behaviors of Antitumor Platinum Drugs with Cucurbiturils
슈퍼관리자
2021-05-21
Theoretical Prediction of the Complexation Behaviors of Antitumor Platinum Drugs with Cucurbiturils
-
Authors :
N. S. Venkataramanan, S. Ambigapathy, H. Mizuseki, and Y. Kawazoe
-
Journal :
J. Phys. Chem. B
-
Vol :
116
-
Page :
14029-14039
-
Year :
2012
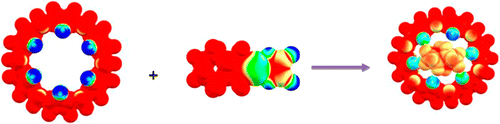
Abstract
The inclusion complex formation ability between CB[n] (n = 6–9) and Pt-drugs (oxaliplatin, nedaplatin, carboplatin, and cisplatin) in gas phase as well as water phases has been investigated using the using density functional theory. The results reveal the existence of several stable inclusion complexes in aqueous solution with high solvation energies compared to the guest and host molecule. It has been shown that the formation of complexes between CB[6] and Pt-drugs resulted in structural change in the CB[6], with the calculated deformation energies being higher for the inclusion complexes. The inclusion complexes are stabilized by the hydrogen bonding and the charge transfer between the Pt-drugs and the CB[n] host. Calculated enthalpy and Gibbs free energy of formation in aqueous solution revels that the formation of CB[7]-oxaliplatin is spontaneous, and hence its experimental synthesis is feasible. Among the CB’s studied, CB[8]–Pt-drug inclusion complexes have exothermic enthalpy and low Gibbs free energy of formation. Computed 1NMR spectra in CB[7]–oxaliplatin showed high chemical shielding for the cyclohexane ring, indicating the existence of charge transfer in the inclusion complex. The amine protons in the guest Pt-drugs are shielded due to the hydrogen bonding interaction with CB’s oxygen portal.















