Electronic Structures and Spectra of Symmetric Meso-Substituted Porphyrin: DFT and TDDFT-PCM Investigations
슈퍼관리자
2021-05-21
Electronic Structures and Spectra of Symmetric Meso-Substituted Porphyrin: DFT and TDDFT-PCM Investigations
-
Authors :
N. S. Venkataramanan, A. Suvitha, H. Nejo, H. Mizuseki, and Y. Kawazoe
-
Journal :
Int. J. Quantum Chem.
-
Vol :
111
-
Page :
2340-2351
-
Year :
2011
Abstract
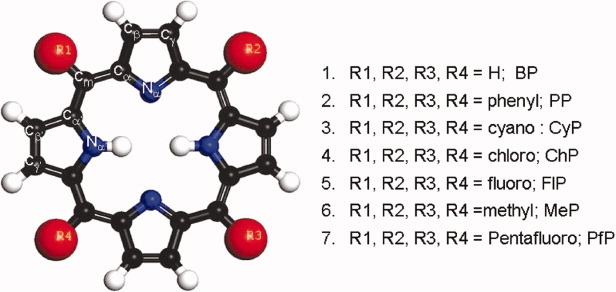
DFT and TDDFT calculations at the level of PBE0/6-31G(d)/6-31+G(d) were performed systematically on seven porphyrins with symmetrical meso-substitutents. Our results show that the planarity of the free base porphyrin (BP) are affected by the introduction of substitutents at the meso-position of the ring. Geometrical studies show that the introduction of electron-withdrawing groups brings about in-plane deformation in the porphyrin ring, whereas the bulky substitutents make an out-of-plane deformation. However, FMO's diagram shows that electron-withdrawing groups alter the degeneracy of the HOMO and HOMO −1 orbtial. Up on introduction of substituents at the meso-position, the Q band FMOs transitions were the same as in the case of free BP; however, the oscillator strength is changed. Electron releasing substituted at the meso-position shows bathochromic shift in the Q band region. However, the intensity or the hyperchromic shift is higher for the electron withdrawing groups. Solvation studies show that Q bands are blue shifted and B bands are red shifted, whereas the intensity of the B bands was highly enhanced compared with the Q bands. These theoretical studies would be helpful in designing new porphyrins for the photodynamic therapy and dye-sensitized solar cell applications. © 2010 Wiley Periodicals, Inc. Int J Quantum Chem, 2011















