Thermodynamic Stability of C3H8 Hydrate of Cubic Structure IV using Lattice Dynamics
슈퍼관리자
2021-05-21
Thermodynamic Stability of C3H8 Hydrate of Cubic Structure IV using Lattice Dynamics
-
Authors :
M. Souissi, R. V. Belosludov, O. S. Subbotin, H. Mizuseki, Y. Kawazoe, and V. R. Belosludov
-
Journal :
J. Incl. Phenom. Macrocycl. Chem.
-
Vol :
69
-
Page :
281-286
-
Year :
2011
Abstract
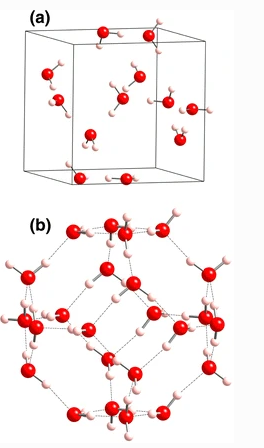
The thermodynamic properties of propane clathrate hydrate with cubic structure IV were studied using a method based on the solid solution theory of van der Waals and Platteeuw but allows one to take into account the influence of guest molecules on the host lattice and guest-guest interactions. The free energies, equations of state, and chemical potentials of this hydrate were estimated using this approach. The proposed theory was used for construction of “guest gas–hydrate–ice I h ” equilibrium curves of propane hydrates. It was found that the water framework of structure IV was dynamically stable and that the fully C3H8 filled structure was thermodynamically stable in the region of pressure from 43 to 50 MPa as compared with the hexagonal ice. The formation pressure of propane hydrate with structure IV is higher than that of propane hydrate with cubic structure II. However, a structural transformation from structure II to IV of propane hydrate was estimated under a pressure of 78 MPa at 290 K.















