Paradox of thiourea: A false-positive and promoter for electrochemical nitrogen reduction on nickel sulfide catalysts
-
Authors :
Min-Cheol Kim, Jiyong Chung, Tae-Yong An, Jaeyoung Lee, Mi-Kyung Han, Shinbi Lee, Wonyong Choi, Jung Kyu Kim, Sang Soo Han, Uk Sim, Taekyung Yu
-
Journal :
Applied Catalysis B: Environmental
-
Vol :
328
-
Page :
122485
-
Year :
2023
Abstract
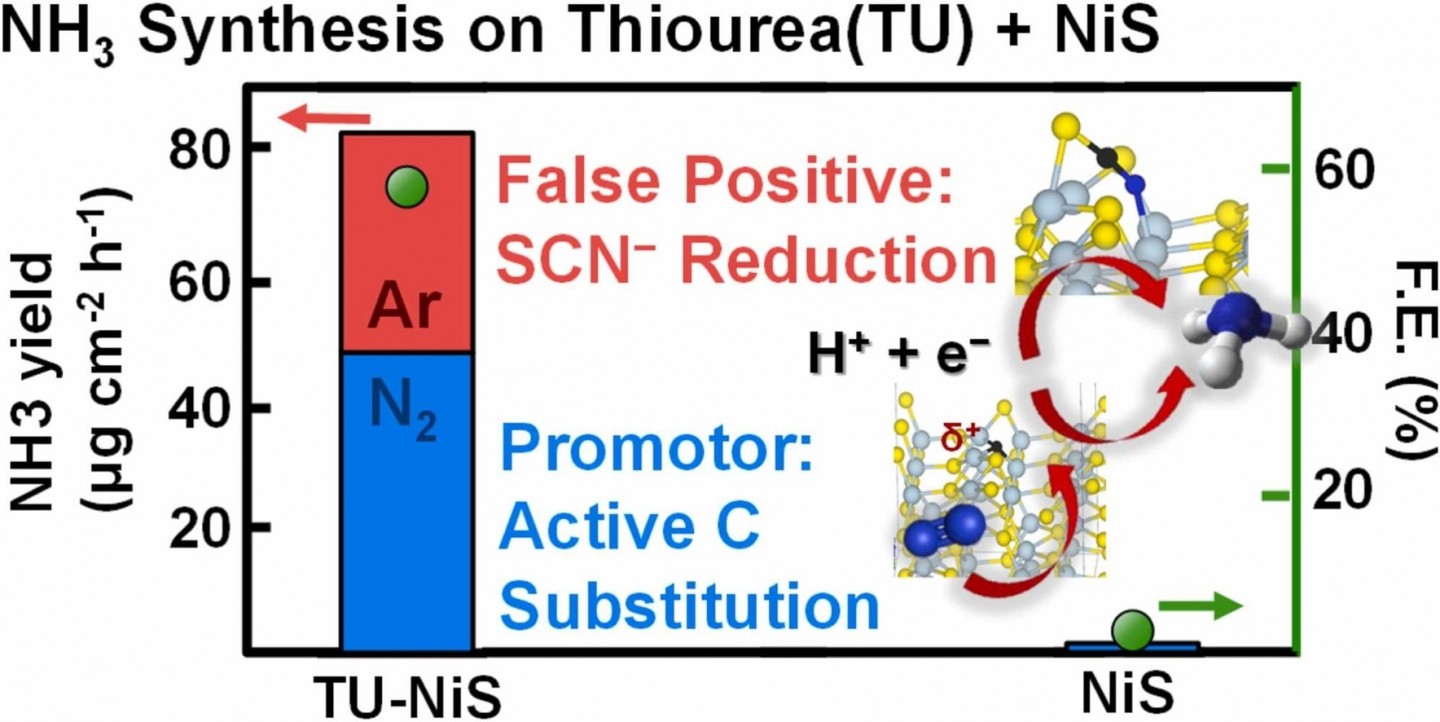
Electrochemical nitrogen reduction reaction (NRR) is an environmentally friendly process for ammonia synthesis. An unusually high NH3 production rate (86.91 ± 9.29 μg h−1 cm−2) and Faradaic efficiency (F.E.) (57.17 ± 4.14 %) was observed in electrochemical tests on NiS catalysts synthesized using thiourea (TU). Through joint experiment-theory work, we demonstrate the paradoxical catalytic effect of TU as a false positive from TU-derived thiocyanate reduction and a promoter via the generation of catalytically active carbon sites after TU reduction on the NiS surface (with an effective NH3 rate of 52 ± 5.57 μg h−1 cm−2). TU reduction leads to the substitution of an S atom to a reactive C atom with fewer valence electrons, facilitating a more facile NRR pathway. This study presents a new possibility for NRR catalysts based on metal-nonmetal compounds (e.g., sulfides, chalcogenides, or nitrides) by nonmetal dopants with fewer valence electrons to create new catalytically active sites.
















