Atomistics of the Lithiation of Oxidized Silicon (SiOx) Nanowires in Reactive Molecular Dynamics Simulations
슈퍼관리자
2021-05-21
Atomistics of the Lithiation of Oxidized Silicon (SiOx) Nanowires in Reactive Molecular Dynamics Simulations
-
Authors :
Hyun Jung, Byung Chul Yeo, Kwang-Ryeol Lee, Sang Soo Han
-
Journal :
Phys. Chem. Chem Phys.
-
Vol :
18
-
Page :
32078-32086
-
Year :
2016
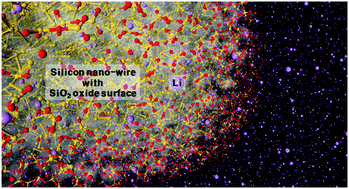
Abstract
Although silicon oxide (SiOx) nanowires (NWs) are recognized as a promising anode material for lithium-ion batteries (LIBs), a clear understanding of their lithiation mechanism has not been reported yet. We elucidate the lithiation mechanism of SiOx NWs at the atomic scale based on molecular dynamics (MD) simulations employing the ReaxFF reactive force field developed through first-principles calculations. SiOx NWs with crystalline Si (c-Si) core and amorphous SiO2 (a-SiO2) shell structures of ∼1 nm in thickness show smaller volume expansion than pristine Si NWs, as found in previous experiments. Lithiation into SiOx NWs creates two interfaces: c-Si/a-LixSi and a-LixSi/a-LiySiO2. The mobility of the latter, which is located farther toward the outside of the NW, is slower than that of the former, which is one of the reasons why the thin SiO2 layer can suppress the volume expansion of SiOx NWs during lithiation. Another reason can be found from the stress distribution, as the SiOx NWs show stress distribution different from the pristine case. Moreover, the lithiation of SiOx NWs leads to the formation of Li2O and Li4SiO4 compounds in the oxide layer, where several Li atoms (not a majority) in Li4SiO4 can escape from the compound and diffuse into the c-Si, in contrast to the Li2O case. However, Li atoms that pass through the SiO2 layer penetrate into the c-Si preferentially along the 〈110〉 or 〈112〉 direction, similar to the mechanism observed in pristine Si NWs. We expect that our comprehensive understanding of the lithiation mechanism of SiOx NWs will provide helpful guidance for the design of SiOx anodes to obtain better performing LIBs.















